During the summer of 2024, representatives of the European Crystallographic Association (ECA) invited me to summarize, in a short video [1], some history published in 2004 [2] and 2015 [3]. The video (Fig. 1) was part of a series, "Women in Crystallography", supported by the Royal Society of Chemistry Inclusion and Diversity Fund. As written in a previous issue of the IUCr Newsletter by the organizers, the video project intends "to celebrate the importance of women in the field of crystallography and science while raising awareness about the pitfalls and obstacles on the way to reach gender parity in science..." [4].
 Figure 1. Opening frame. Women in Crystallography #5. Produced by the European Crystallographic Association [1].
Figure 1. Opening frame. Women in Crystallography #5. Produced by the European Crystallographic Association [1].
Since then, in the United States, considerations of diversity, equity and inclusion (DEI) in science, education and the workplace, once welcomed by the government, have been reframed, foremost in executive orders (nos. 14151 and 14173) in January 2025 - "Ending Radical and Wasteful Government DEI Programs and Preferences" and "Ending Illegal Discrimination and Restoring Merit-Based Opportunity" [5]. However, the US National Science Foundation (NSF) supported the research that informed the video production under its Broader Impacts mission. For more than three decades, the NSF has doggedly forged a scientific workforce that more closely represents our multi-gendered multicultural American society by enlarging the concept of merit. In the sincerity and tenacity of this ongoing effort, the NSF has transformed the national academic science community. These NSF values on which I was raised as a scientist are well aligned with those of the ECA. However, this alignment will undoubtedly require increasing chiropractic care as the United States dismisses common interests with other nations and broadcasts its new policies that label concerns about representation as misguided and disqualifying.
Long before this new dark age, as I was preparing to attend university, my older brother announced that he would go first and study psychology. "A fool's mission", I declared, "People are too complicated. I will study crystals", I predicted with satisfaction. We did as we said. Now, decades later, it seems obvious to me that our greatest problems as a community will not be solved with crystals if we don't first understand more about human behavior. Perovskite solar cells must be directed at the sun…by people. Despite raising a thousand generations of babies since neolithic times, we cannot eschew the individuals among us who may affect, directly and adversely, millions.
The video requested by the ECA was an effort to explain something about people, still too complicated but perhaps not hopelessly so.
It has been observed frequently that women were atypically well populated in one area of physical science in the last century - the study of crystal structure with X-rays (see e.g. [6]). Dorothy Hodgkin (1910-1994), Kathleen Lonsdale (1903-1971), Dorothy Wrinch (1894-1976), Elizabeth Wood (1912-2006), Helen Megaw (1907-1922) and Rosalind Franklin (1920-1958), stand out, among others. Since this group was such a bright spot in an otherwise male-centric history of physical science, it invited explanations. But, because of a lack of understanding of psychology, these purported explanations have tended to be pejorative. Some have said that crystallography was a helpmate to chemistry, physics, biology and geology, just like wives were traditionally helpmates to husbands. Some have said that summing terms in a Fourier series prior to electronic computers was tedious, like knitting, and well adapted to the female mind. These cavalier "explanations" were collected in [2].
However, there came into my focus a credible evidence-based understanding of this gender anomaly. It came into my focus only because I was engaged in the business of raising one of those post-neolithic babies. Only after sending a child to kindergarten did I learn what kindergarten had been all about at the time of its inception. Moreover, the foundational principles of kindergarten, improbably, impacted the early educational experiences of the pioneering X-ray crystallographers, a story that I was asked to tell by members of the ECA, using the medium of film actualized by the aforementioned kindergartener, now a grown-up filmmaker. After our contribution to the ECA project was completed, the IUCr Newsletter Editor invited me to reduce the film to this article. So, here is a story about inclusivity, told with the confidence that these stories remain valuable.
Crystallography, it turns out, had a tremendous influence on early childhood education in the 19th century generally. We became aware of this through the work of two scholars: Jeanne Rubin, a music professor at Kent State University [7] and Norman Brosterman, an architect and historian [8]. They told the story of Friederich Fröbel (1782-1852) (scholarship on the influence of crystallography on kindergarten continues, in particular by examination of Fröbel’s notebooks held in the Fröbel museum in Bad Blankenburg, see e.g. [9a, 9b]).
Fröbel was born in the Thuringian forest of Prussia. He was largely self-educated but took some natural science classes in Jena and Göttingen, interrupted by a stay in a debtor's prison and his voluntary service battling Napoleon's army. Afterward, somehow, he became an assistant to Christian Samuel Weiss (1780-1856) at the Mineralogy Museum in Berlin. Weiss invented the concept of the crystallographic system. Fröbel must have been a favored apprentice because, in 1817, he was offered a professorship in mineralogy in Stockholm. He reluctantly turned it down.
Fröbel, a 19th-century romantic, believed children and crystals grow according to the same natural laws. However, he thought children were too complicated to study, a fool's mission. On the other hand, in the 19th century, crystals were merely polyhedra that could be described with geometry. Fröbel thus believed that the surest path for children to the mind of God was the study of crystals and their symmetries. Fröbel explained how this notion caused him to depart from his career plans in higher education. He said [reduced for concision]:
"It had long been my dearest wish to devote myself to an academic career, for I thought to find in it my vocation, the meaning of my life. But the opportunity to get to know students and … their lack of any true scientific spirit made me go back on my purpose ... Meanwhile, the stones in my hand and under my eyes became forms of life that spoke a language I understood. The world of crystals clearly proclaimed the structure of life to me" [10,11].
Fröbel devoted the rest of his life to developing a system of early childhood education. In Prussia, in the early 19th century, schooling typically began at age 7. To seize on lost opportunity, Fröbel's invented kindergarten [8] for younger children with a highly circumscribed pedagogy. The Fröbel kindergarten curriculum was based on 20 so-called Gifts and Occupations. Each were lattice, tessellation or polyhedron-building exercises of one kind or another so that children could mimic crystals with a dual educational and spiritual purpose. The "polymorphism" arising from the decoration of "special positions" with tiles or blocks having the same symmetry as the ensemble was a staple of Fröbel's training, often on gridded tabletops (Fig. 2, [12,13]). Stepwise instructions for the transformation of these iso-symmetric forms are given in a recent book by Rogers [14].
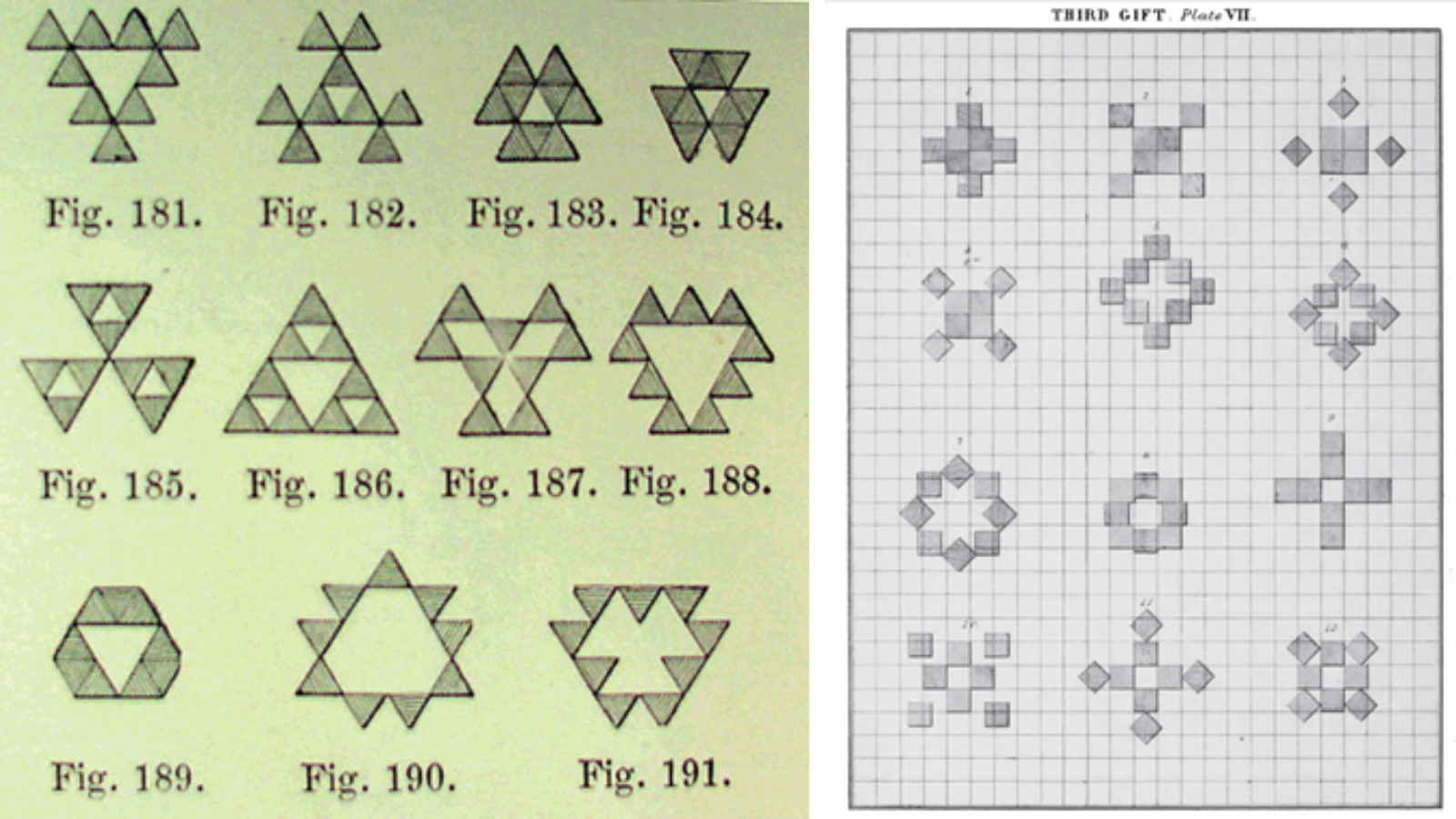 Figure 2. Activities with 9 equilateral triangular tiles (left) and 8 cubic blocks (right) from manuals for kindergarten teachers, [11] and [12], respectively.
Figure 2. Activities with 9 equilateral triangular tiles (left) and 8 cubic blocks (right) from manuals for kindergarten teachers, [11] and [12], respectively.
Following the pro-democracy rebellions that rocked Europe in 1848, the Prussian monarchy decided it was safer if the people remained undereducated. Kindergarten was outlawed, and Fröbel died heartbroken. But, by this time, talented women had taken to teaching kindergarten as a vocation, and Fröbel's invention spread quickly internationally [15]. However, its crystallographic content was diminished by successive generations because new kindergarten teachers were not trained as mineralogists.
Jeanne Rubin lived in a house designed by the pioneering American architect Frank Lloyd Wright (1867-1959), and that is how her book Intimate Triangle [7] joins Wright to the two mineralogists Weiss and Fröbel on its cover (Fig. 3). Wright's mother, Anna, was a Fröbel kindergarten teacher in Wisconsin, and Wright wrote in his autobiographies [16a, 16b] about how important old Fröbel's philosophy was to his development as an architect.
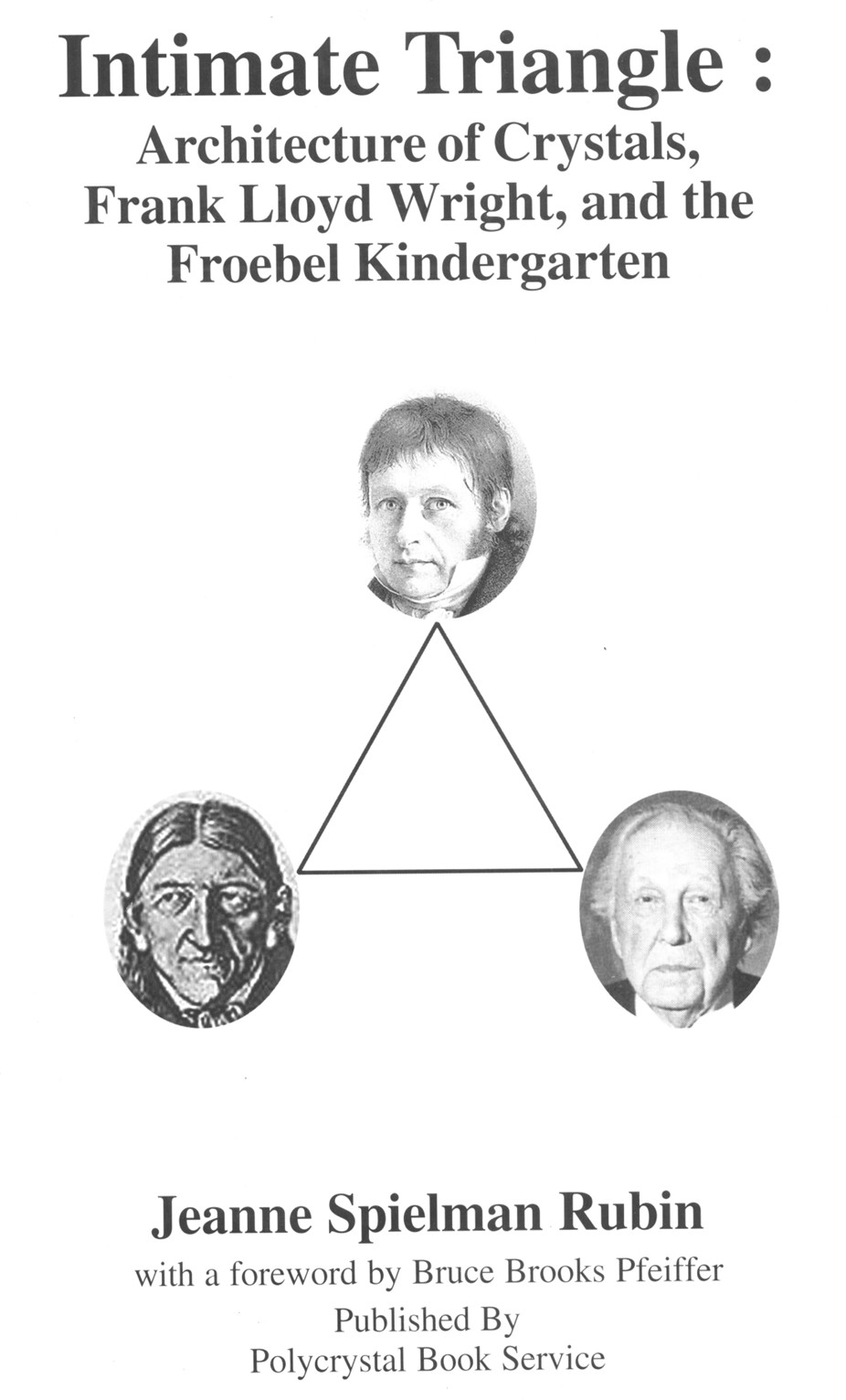 Figure 3. Book jacket [7]. Top: Christian Samuel Weiss. Right: Frank Lloyd Wright. Left: Friederich Fröbel.
Figure 3. Book jacket [7]. Top: Christian Samuel Weiss. Right: Frank Lloyd Wright. Left: Friederich Fröbel.
However, Rubin and Brosterman show through their scholarship and the unusual hobby of Brosterman - collecting vintage kindergarten art - that the influence of Fröbel kindergartens extended well beyond Wright. Legions of children in the half-century preceding World War I were exposed to Fröbel's methodology. Rubin and Brosterman persuasively argue that the Fröbel system of training children to work with the geometries and symmetries of crystals was a force that led to modernism in the visual arts and architecture brought forward by Fröbel kindergarten graduates. Besides Wright, Le Corbusier (1887-1965), Paul Klee (1979-1940), Walter Gropius (1883-1969), Josef Albers, Wassily Kandinsky (1888-1976) and Georges Braque (1882-1963), among other pioneers, likely attended Fröbel or Fröbel-inspired kindergartens. Brosterman's comparisons [8] of turn-of-the-century American kindergarten art with paintings by famous artists make a compelling case for a Fröbel-catalyzed rebellion against convention in Western art and architecture.
In reviewing this history, we articulated a natural corollary:
"If many of the pioneering artists had their geometric sensibilities inculcated in Fröbel kindergartens, it is likely that pioneering X-ray crystallographers had a similar experience. Did the crystallographic content of late nineteenth-century kindergarten play a role in the explosion of research into the architecture of crystals? In other words, did the crystallographic content of kindergarten influence the future development of crystallography?" [2].
Such an investigation would require a collection of early childhood biographies of the pioneers of X-ray crystallography. A place to start would be with the women especially. Is it possible that many girls were exposed to crystallography in kindergarten before being systemically shut out of the study of the natural sciences by conventional schooling biases?
Our friend Jeanne Rubin took up this suggestion. She wrote by email in 2006 [17]: "On your question about the burgeoning number of female crystallographers early in the last century, I've already tracked down documentation of the Fröbel training of Rosalind Franklin and am working on others." However, the next year, Jeanne passed away at the age of 90.
Years later, in 2015, there was an invitation to contribute to a special issue of the American Chemical Society journal Crystal Growth and Design organized in memory of the widely admired crystallographer Peggy Etter (1943-1992) of the University of Minnesota. For this initiative, we thought that we should try to finish the inquiry that Rubin started. Much had changed since 2006. The adolescent internet had grown up. While it would have been impossible to unearth the philosophies of the educational experiences of the pioneering female X-ray crystallographers in the early years of the 20th century, the web had spread quickly and we could follow its tendrils.
Rosalind Franklin's first school was Norland Place, founded by Emily Lord (1850-1930) to spread Fröbel's philosophy to kids aged 3-8. Lord founded Britain's Fröbel society. Dorothy Hodgkin attended the Parents National Educational Union founded by Charlotte Mason (1842-1923), a Fröbel disciple who said "We reverence Friederich Fröbel. We share many of his great thoughts.” According to Hodgkin, "[The school] produced small books to introduce their pupils to the different sciences. The book on chemistry began with growing crystals of copper sulfate and alum. I found this fascinating and repeated the experiments at home." Elizabeth Wood attended the Horace Mann School, an experimental high school of Columbia University's Teacher's College. The Teacher's College Record put out a stream of articles on the virtues of Fröbel schooling from 1900 onwards. Horace Mann (1796-1859) was the brother-in-law of Elizabeth Peabody (1808-1894), for whom the Fröbel kindergarten "was not simply a method of education but a movement of mystical significance. Her advocacy was an 'apostolate', kindergartening a religion, a 'Gospel for children'." Mary Peabody Mann (1806-1887), the wife of Horace, translated [15] from German to English. Mary called the Fröbel kindergarten "the free republic of childhood" [15, p. ix]. Helen Megaw attended the Alexandra School in Dublin that cites a "philosophy of teaching and learning [that] is child-centered and based on the principles of Friedrich Fröbel". The Alexandra School worked with the Fröbel College in Sion Hill to extend Fröbel pedagogy to the lower classes. Later, she attended Roedean School in Brighton, founded by Penelope Lawrence (1856-1932). Lawrence was the principal of the Fröbel Society's Kindergarten Training College in London. Kathleen Lonsdale attended the Bedford Training College, which prepared students for National Fröbel Union Examinations. Dorothy Wrinch's sister Muriel earned a Fröbel certificate. She attended Girton College in Cambridge, where her headmistress was Emily Shirreff (1814-1897), founder of the Fröbel Society in 1876. (The citations that support each attribution and quotation in this paragraph can be found in [3].)
The United States aspires to strengthen its scientific workforce by ensuring that it more closely reflects the genders and ethnicities of the American population. Activities that support this democratic goal are said to have "Broader Impacts" because they align science and engineering with societal goals. But, societal goals seem to be changing, rapidly. The NSF's Broader Impacts landing page at present [18], by happenstance, has a banner that features some crystals grown in our laboratory. Creating a "diverse" workforce remains one of the ways advertised to satisfy the Broader Impacts mission, even while webpages (.gov) of federal agencies in the USA are being scrubbed (last examined 29 March 2025).
Psychologists and sociologists have considered the importance of representation, and we should know what they have learned ([19], see examples and references therein). Sustained achievement in a field filled with obstacles, both intrinsic to the discipline and extrinsic from prejudicial expectations of others, requires identification with the discipline. The cultivation of an identity associated with science is the counterweight to stereotype threats, malicious or otherwise. People will fight to maintain their identities, even when the going gets tough. Where better than kindergarten to forge an identity with science and mathematics that can serve to counteract the negative consequence of prejudice? This is what Friederich Fröbel achieved through kindergarten.
Naturally, if the Fröbel kindergarten had influenced girls, it would have also worked on boys. For instance, we don't know much about the early life of the idiosyncratic William Barlow (1845-1934), a pre-X-ray pioneer in crystal structure and space group prediction [20]. Barlow, an heir to a sizeable estate, didn't need or receive a systematic education, but he approached crystals systematically. Was he imprinted by a crystallographic system at a formidable age? Barlow turned 6 when Johannes (1813-1887) and Bertha Ronge (1818-1863) brought the first Fröbel kindergarten to Britain [13]. Their school was sited in central London, where Barlow was born and raised. Are there identifiable reasons for the devotion of Barlow, among others, to the internal structure of crystals? We haven't collected the necessary biographical facts, and boys may have been encouraged by more obvious mechanisms. Crystallography may also have been welcoming to young women because of the egalitarian impulses of William Henry Bragg, William Lawrence Bragg and John Desmond Bernal. There won't be a universal explanation.
Did X-ray crystallography receive a special boost in female contributions because of the crystallographic pedagogy of the Fröbel kindergarten? Perhaps. But establishing causality in history is hard. In crystallography, we like statistics. But lack of certainty is preferable to silly comparisons of X-ray analysis to knitting. Meanwhile, check out the other ECA videos in the series here.
References
[1] European Crystallographic Association (2025). Fifth video of the “Women in Crystallography” series, https://ecanews.org/blog/2025/03/07/fifth-video-of-the-women-in-crystallography-series/.
[2] Kahr, B. (2004). Crystal engineering in kindergarten. Cryst. Growth Des. 4, 3–9.
[3] Kahr, B. (2015). Broader impacts of women in crystallography. Cryst. Growth Des. 15, 4715–4730.
[4] Women in Crystallography project (2024). IUCr Newsletter, 32, https://www.iucr.org/news/newsletter/volume-32/number-2/women-in-crystallography-project.
[5] National Archives (2025). Executive Orders, https://www.federalregister.gov/presidential-documents/executive-orders/.
[6] Aparicio, J. S. (2015). The legacy of women to crystallography, Arbor, 191, a216.
[7] Rubin, J. S. (2002). Intimate triangle: architecture of crystals, Frank Lloyd Wright and the Froebel kindergarten. Huntsville, Alabama: Polycrystal Book Service.
[8] Brosterman, N. (1997). Inventing kindergarten. New York: Harry N. Abrams.
[9a] Friedman, M. & Muñoz Alvis, J. (2023). Haüy, Weiß, Fröbel: the influence of nineteenth-century crystallography on the mathematics of Friedrich Fröbel's kindergarten. Part 1: the published materials. Paedagog. Hist. 59, 191–211.
[9b] Friedman, M. & Muñoz Alvis, J. (2023). Haüy, Weiß, Fröbel: the influence of nineteenth-century crystallography on the mathematics of Friedrich Fröbel's kindergarten. Part 2: new evidence from unpublished notes. Paedagog. Hist. 59, 212–232.
[10] Froebel, F. (1967). Letter to Karl Christoph Friederich Krause, 1828. Friederich Froebel: a selection from his writings, edited by I. M. Lilley, p. 39. Cambridge University Press.
[11] Froebel, F. (1906). Autobiography of Friederich Froebel, p. 112. London: Swan Sonnenschein & Co.
[12] Wiebé, E. (1910). Golden jubilee edition of the paradise of childhood. Springfield: Milton Bradley.
[13] Ronge, J. & Ronge, B. (1858). Practical guide to English kindergarten, 2nd ed. London: Hodson and Son.
[14] Rogers, W. (2016). Close-up view of Froebel's kindergarten with Frank Lloyd Wright at the drawing table. Xlibris.
[15] von Marenholtz-Bülow, B. (2007). How kindergarten came to America. New York: The New Press. [Originally published in 1895 as The reminiscences of Friederich Froebel. Boston: Lee and Shepard.]
[16a] Wright, F. L. (1943). An Autobiography, pp. 13-14. New York: Duell, Sloan and Pearce.
[16b] Wright, F. L. (1957). A Testament, pp. 19-21, 63, 100, 206-207, 220. New York: Bramhall House.
[17] Rubin, J. (2006). Email to B. Kahr, 10 April.
[18] National Science Foundation (2025). Broader impacts, https://www.nsf.gov/funding/learn/broader-impacts.
[19] Steele, C. M. (2011). Whistling Vivaldi. New York: W. W. Norton.
[20] Tandy, P. (2004). William Barlow (1845–1934): speculative builder, man of leisure and inspired crystallographer. Proc. Geol. Assoc. 115, 77–84.
Bart Kahr is located at New York University in New York City.


![[Cover v25n2]](https://www.iucr.org/__data/assets/image/0018/135180/cover25n2.jpg)






 Figure 1. George M. Sheldrick and the diffractometer Quatermass (in the background), which was designed and built from components from many manufacturers to boost its functionality. George had a broad interest in science and occupied his bright mind with problem solving in structural chemistry. Possibly, he was a scientist from his birth in Huddersfield, Yorkshire. He was certainly a chess player before becoming a chemist.
Figure 1. George M. Sheldrick and the diffractometer Quatermass (in the background), which was designed and built from components from many manufacturers to boost its functionality. George had a broad interest in science and occupied his bright mind with problem solving in structural chemistry. Possibly, he was a scientist from his birth in Huddersfield, Yorkshire. He was certainly a chess player before becoming a chemist.
 Figure 2. Cruickshank Symposium, UMIST, in September 1984. Front row, from left to right: Professor J. D. Dunitz FRS, Professor G. A. Jeffrey, Professor Dorothy Hodgkin FRS, Professor D. W. J. Cruickshank FRS, Professor Mary R. Truter. Behind: Professor K. N. Trueblood, Professor F. L. Hirshfeld, Dr J. S. Rollett, Professor J. E. Boggs, Professor A. C. T. North, Professor O. Bastiansen, Dr G. S. Pawley, Dr J. R. Helliwell, Professor G. M. Sheldrick (FRS 2001).
Figure 2. Cruickshank Symposium, UMIST, in September 1984. Front row, from left to right: Professor J. D. Dunitz FRS, Professor G. A. Jeffrey, Professor Dorothy Hodgkin FRS, Professor D. W. J. Cruickshank FRS, Professor Mary R. Truter. Behind: Professor K. N. Trueblood, Professor F. L. Hirshfeld, Dr J. S. Rollett, Professor J. E. Boggs, Professor A. C. T. North, Professor O. Bastiansen, Dr G. S. Pawley, Dr J. R. Helliwell, Professor G. M. Sheldrick (FRS 2001).
 Figure 3. It was in Cambridge where George met the love of his life, Katherine Elizabeth Herford. They were married in Jesus College on 13 July 1968.
Figure 3. It was in Cambridge where George met the love of his life, Katherine Elizabeth Herford. They were married in Jesus College on 13 July 1968.
 Figure 4. From the start, SHELX was user-friendly: well documented and supported. On submitting this manuscript, SHELX-76 was downloaded from the IUCr website
Figure 4. From the start, SHELX was user-friendly: well documented and supported. On submitting this manuscript, SHELX-76 was downloaded from the IUCr website  Figure 5. Daily meetings and occasional visitors around the coffee table. From left to right: Frank Pauer, Sally Brooker, Thomas Kottke, Ursula Pieper, Kirsten Krahnstöver, Axel Göhrt, Regine Herbst-Irmer, Holger Beck, Erhard Irmer, George Sheldrick.
Figure 5. Daily meetings and occasional visitors around the coffee table. From left to right: Frank Pauer, Sally Brooker, Thomas Kottke, Ursula Pieper, Kirsten Krahnstöver, Axel Göhrt, Regine Herbst-Irmer, Holger Beck, Erhard Irmer, George Sheldrick.
 Figure 6. Group in the early 1990s around the Quatermass diffractometer. Back row left to right: Heinz Gornitzka, Dietmar Stalke, Ludger Häming, George M. Sheldrick, Alexander Steiner, Helmut Dehnhardt, Annja Kuhn, Regine Herbst-Irmer, Andreas Heine. Front row: Ehmke Pohl, Miene Schäfer, Ursula Pieper.
Figure 6. Group in the early 1990s around the Quatermass diffractometer. Back row left to right: Heinz Gornitzka, Dietmar Stalke, Ludger Häming, George M. Sheldrick, Alexander Steiner, Helmut Dehnhardt, Annja Kuhn, Regine Herbst-Irmer, Andreas Heine. Front row: Ehmke Pohl, Miene Schäfer, Ursula Pieper.
 Figure 7. George and his group at the ACA 2011. Left to right: Birger Dittrich, Christian Hübschle, Peter Müller, Kevin Pröpper, George M. Sheldrick accompanied by Katherine E. Sheldrick.
Figure 7. George and his group at the ACA 2011. Left to right: Birger Dittrich, Christian Hübschle, Peter Müller, Kevin Pröpper, George M. Sheldrick accompanied by Katherine E. Sheldrick.




 Figure 1. Opening frame. Women in Crystallography #5. Produced by the European Crystallographic Association [1].
Figure 1. Opening frame. Women in Crystallography #5. Produced by the European Crystallographic Association [1].
 Figure 2. Activities with 9 equilateral triangular tiles (left) and 8 cubic blocks (right) from manuals for kindergarten teachers, [11] and [12], respectively.
Figure 2. Activities with 9 equilateral triangular tiles (left) and 8 cubic blocks (right) from manuals for kindergarten teachers, [11] and [12], respectively.
 Figure 3. Book jacket [7]. Top: Christian Samuel Weiss. Right: Frank Lloyd Wright. Left: Friederich Fröbel.
Figure 3. Book jacket [7]. Top: Christian Samuel Weiss. Right: Frank Lloyd Wright. Left: Friederich Fröbel.