Martian crystallography
Water-rich halide hydrate structures have not grabbed the attention of chemists ... until very recently when it was realized that such ionic compounds might create life-bearing aqueous pools temporarily at temperatures as low as 200 K and that such systems might exist on the chilly surface of the planet Mars.
Over the last few years various craft sent to Mars - Viking, Phoenix and Curiosity - have hinted at the chemistry of the Red Planet. Large quantities of salts, including sulfates and chlorides, as well as water droplets have been detected, and geological evidence for aqueous processes includes valleys and slopes that are clearly visible today.
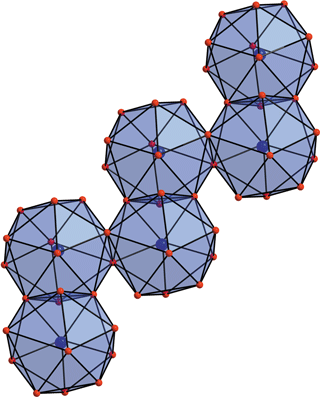 Writing in Acta Crystallographica [Schmidt et al. (2014). Acta Cryst. C70, 882-888; doi:10.1107/S2053229614014302], inorganic chemists Horst Schmidt, Erik Hennings and Wolfgang Voigt of TU Bergakademie Freiberg, Germany, have crystallized five interesting hydrates: the nonahydrate of aluminium bromide; the stable pentadecahydrates of aluminium chloride, bromide and iodide; and a metastable heptadecahydrate of the iodide from low-temperature solutions. They have determined the crystal structures of these compounds as part of their ongoing work investigating the crystallization and dissolution close to the lower-temperature extremes observed on Mars.
Writing in Acta Crystallographica [Schmidt et al. (2014). Acta Cryst. C70, 882-888; doi:10.1107/S2053229614014302], inorganic chemists Horst Schmidt, Erik Hennings and Wolfgang Voigt of TU Bergakademie Freiberg, Germany, have crystallized five interesting hydrates: the nonahydrate of aluminium bromide; the stable pentadecahydrates of aluminium chloride, bromide and iodide; and a metastable heptadecahydrate of the iodide from low-temperature solutions. They have determined the crystal structures of these compounds as part of their ongoing work investigating the crystallization and dissolution close to the lower-temperature extremes observed on Mars.The three pentadecahydrates of aluminium chloride, bromide and iodide represent the most water-rich hydrates known for aluminium salts, the team says. The team explains that the development of cation hydration spheres in these structures is critical. They found that the pentadecahydrate of the chloride and bromide are isostructural. In the iodide species, half of the Al cations are surprisingly surrounded by two complete hydration spheres, with six water molecules in the primary sphere and twelve in the secondary. And, in the heptadecahydrate of aluminium iodide, this level of hydration was seen with every single Al3+. Indeed, the heptadecahydrate, AlI3.17H2O, is the most water-rich hydrate so far prepared from an aluminium salt, the team reports. However, it crystallizes only as an intermediate phase that changes habit and composition within days to form a pentadecahydrate.
"When freezing-thawing cycles at low temperatures play a role in water redistribution on Mars then one has to expect other enrichments of the elements than on Earth," team member Wolfgang Voigt told us. "The high concentration of perchlorate was the first surprise, others might follow. Further work is directed at developing a general understanding of the crystallization of salt hydrates at low temperatures with halides, perchlorates and sulfates being the focus."


