Protein
microcrystallography using synchrotron radiation
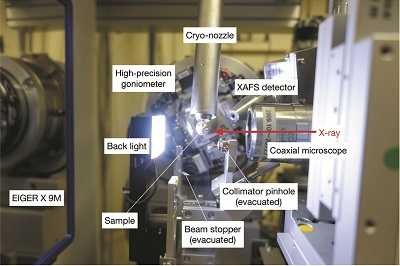
The progress in X-ray microbeam applications using
synchrotron radiation is beneficial to structure determination from
macromolecular microcrystals such as small in meso crystals. However, the high
intensity of microbeams causes severe radiation damage, which worsens both the
statistical quality of diffraction data and their resolution, and in the worst
cases results in the failure of structure determination. Even in the event of
successful structure determination, site-specific damage can lead to the
misinterpretation of structural features. In order to overcome this issue,
technological developments in sample handling and delivery, data-collection
strategy and data processing have been made. For a few crystals with dimensions
of the order of 10 µm, an elegant two-step scanning strategy works well. For
smaller samples, the development of a novel method to analyze multiple
isomorphous microcrystals was motivated by the success of serial femtosecond
crystallography with X-ray free-electron lasers. This method overcame the
radiation-dose limit in diffraction data collection by using a sufficient
number of crystals. In
Yamamoto,
M. et al. (2017). IUCrJ, 4, 529-539 important technologies and the future
prospects for microcrystallography are discussed.
 The progress in X-ray microbeam applications using
synchrotron radiation is beneficial to structure determination from
macromolecular microcrystals such as small in meso crystals. However, the high
intensity of microbeams causes severe radiation damage, which worsens both the
statistical quality of diffraction data and their resolution, and in the worst
cases results in the failure of structure determination. Even in the event of
successful structure determination, site-specific damage can lead to the
misinterpretation of structural features. In order to overcome this issue,
technological developments in sample handling and delivery, data-collection
strategy and data processing have been made. For a few crystals with dimensions
of the order of 10 µm, an elegant two-step scanning strategy works well. For
smaller samples, the development of a novel method to analyze multiple
isomorphous microcrystals was motivated by the success of serial femtosecond
crystallography with X-ray free-electron lasers. This method overcame the
radiation-dose limit in diffraction data collection by using a sufficient
number of crystals. In Yamamoto,
M. et al. (2017). IUCrJ, 4, 529-539 important technologies and the future
prospects for microcrystallography are discussed.
The progress in X-ray microbeam applications using
synchrotron radiation is beneficial to structure determination from
macromolecular microcrystals such as small in meso crystals. However, the high
intensity of microbeams causes severe radiation damage, which worsens both the
statistical quality of diffraction data and their resolution, and in the worst
cases results in the failure of structure determination. Even in the event of
successful structure determination, site-specific damage can lead to the
misinterpretation of structural features. In order to overcome this issue,
technological developments in sample handling and delivery, data-collection
strategy and data processing have been made. For a few crystals with dimensions
of the order of 10 µm, an elegant two-step scanning strategy works well. For
smaller samples, the development of a novel method to analyze multiple
isomorphous microcrystals was motivated by the success of serial femtosecond
crystallography with X-ray free-electron lasers. This method overcame the
radiation-dose limit in diffraction data collection by using a sufficient
number of crystals. In Yamamoto,
M. et al. (2017). IUCrJ, 4, 529-539 important technologies and the future
prospects for microcrystallography are discussed.


