Radiation damage in a nucleoprotein complex
 Radiation
damage has been a curse of macromolecular crystallography from its early days
but recent work to systematically quantify its effect on nucleoprotein complexes suggests that RNA may protect
these complexes [Dauter et al.
(2016). Acta Cryst. D72, 601-602; doi:10.1107/S2059798316006550].
Radiation
damage has been a curse of macromolecular crystallography from its early days
but recent work to systematically quantify its effect on nucleoprotein complexes suggests that RNA may protect
these complexes [Dauter et al.
(2016). Acta Cryst. D72, 601-602; doi:10.1107/S2059798316006550].
The problem of radiation damage was very acute when diffraction data were measured from crystals kept at ambient temperatures. The introduction of cryo-cooling techniques to some extent alleviated the severity of the damaging effects incurred by protein and nucleic acid crystals, but the very intense synchrotron sources now used may destroy diffracting crystals after minutes or seconds of exposure. Not only does the quality of diffraction data and structure solution processes suffer, but, more importantly, radiation damage may lead to misinterpretation of chemical and biological results and to false mechanistic conclusions. Radiation damage has thus become a hot topic of contemporary macromolecular methodology; dedicated international workshops are held every two years and the proceedings have been published in the Journal of Synchrotron Radiation.
The effects of radiation damage are manifested globally as a decrease in the total crystal diffraction power, a change of unit-cell dimensions, an increase of crystal mosaicity or eventually its cracking and disintegration. However, even after absorbing smaller energy doses, many specific local effects of damage can be identified within the structures of macromolecules.
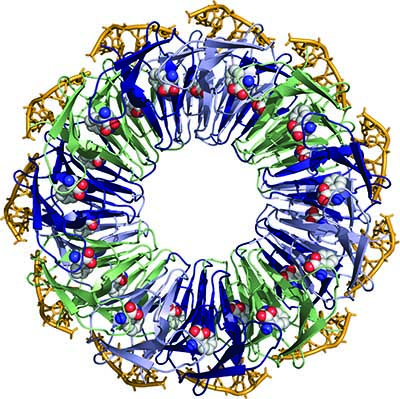
Particularly active is this field is the group at the University of Oxford headed by Elspeth Garman. In a recent paper [Bury et al. (2016). Acta Cryst. D72, 648-657; doi:10.1107/S2059798316003351] this group and their collaborators describe an ingenious method to systematically quantify the effect of increasing absorbed dose on individual atoms of the structure, and then apply it to a crystal structure containing simultaneously an un-complexed protein and its complex with RNA.
Over a large dose range, the RNA was found to be far less susceptible to radiation-induced chemical changes than the protein. Unexpectedly, the RNA binding was observed to protect otherwise highly sensitive residues within the RNA-binding pockets distributed around the outside of the protein molecule. Additionally, the method enabled a quantification of the reduction in radiation-induced disordering upon RNA binding, directly from the electron density.
The paper thus presents a novel objective methodology for judging the effects of radiation damage on macromolecular crystals that will certainly be extremely helpful for the community of macromolecular crystallographers.


