Rising to the challenge of SAD phasing for SFX
Serial femtosecond crystallography (SFX) using ultrashort pulses from X-ray free-electron lasers has become an area of intense investigation. In particular, SFX takes advantage of the acquisition of high-resolution protein structures by the 'diffraction-before-destruction' approach and has successfully been used to collect data from small protein crystals at the micrometre to submicrometre scale. This technique thus removes the bottleneck of obtaining crystals of sufficient size for conventional data collection, and allows structures to be determined from small crystals with minimal radiation damage.
Until now, however, structure determination using de novo phasing of native proteins has not been very common, and the molecular-replacement method (which relies on previous knowledge of related known structures) or heavy-atom derivatives of protein crystals have largely been used in SFX.
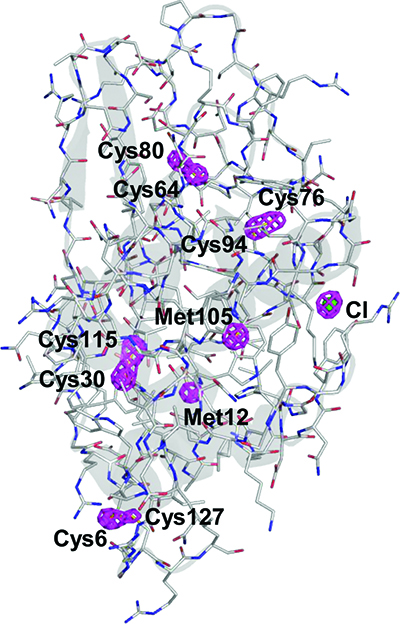
A group of scientists in Japan has now demonstrated the successful phasing of native proteins in SFX by applying a sulfur/chlorine single-wavelength anomalous diffraction (SAD) method [Nakane et al. (2015). Acta Cryst. D71, 2519-2525; doi: 10.1107/S139900471501857X]. This approach does not require the preparation of a heavy-atom derivative, which is usually a difficult task with unreliable results, or the knowledge of a related structure. In contrast, the SAD method uses the anomalous signal arising from the ubiquitous S atoms in native proteins to solve the crystallographic phase problem. This technique thus represents an important milestone in the rapidly developing field of SFX.


