


Meeting report

Two New Protein Folding Patterns Revealed
The highlights of the 1993 meeting of the American Crystallographic Association in Albuquerque, NM, USA, May 21-28, 1993 included reports of exciting advances in theory, instrumentation, and application of crystallography, a superb Patterson Award lecture by G. Sheldrick, the largest most successful poster session in ACA history, a vibrant exhibition of the latest in equipment and supplies, and an immensely popular commemorative T-shirt that sets a new standard for the ACA.
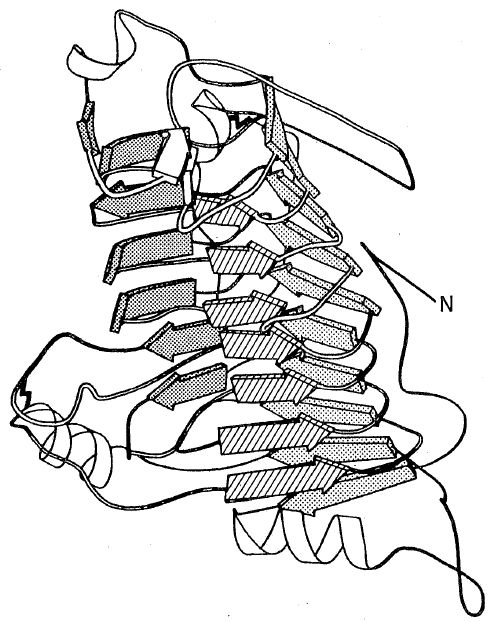 Ribbon diagram illustrating parallel β-sheet coil observed in pectate lyase C (Yoder, M., Keen, N., and Jarnach, F., Abstract D017, pg. 34, ACA Ab., Series 2, Vol. 21, May 1993, Albuquerque, NM. Figure created with the program RIBBONS (M. Carsons, U. AL). Photo courtesy of M. Yoder.
Ribbon diagram illustrating parallel β-sheet coil observed in pectate lyase C (Yoder, M., Keen, N., and Jarnach, F., Abstract D017, pg. 34, ACA Ab., Series 2, Vol. 21, May 1993, Albuquerque, NM. Figure created with the program RIBBONS (M. Carsons, U. AL). Photo courtesy of M. Yoder.
Of particular note were reports of two entirely new protein folding patterns found in macromolecular structures. M. Yoder's Monday morning description of a coiled β-sheet in the structure of pectate lyase C electrified the audience of 300 macromolecule crystallographers. The horseshoe-shaped arrangement of alternate β-strands and α-helices discovered in the structure of a porcine ribonuclease inhibitor with leucine-rich repeats reported by B. Kobe and J. Deisenhofer was equally remarkable. Although there are over 1,110 protein crystal structures in the Brookhaven Protein Data Bank they represent a small fraction of the total proteins in the ecosystem. Due to special (if not unique) properties of individual proteins that hinder efforts to obtain suitable crystals, the data base does not contain examples of many principal types of protein folds. The fact that two entirely new folds and tertiary structures have been discovered illustrates anew how vital macromolecular crystal structure analysis is to the field of protein biochemistry.
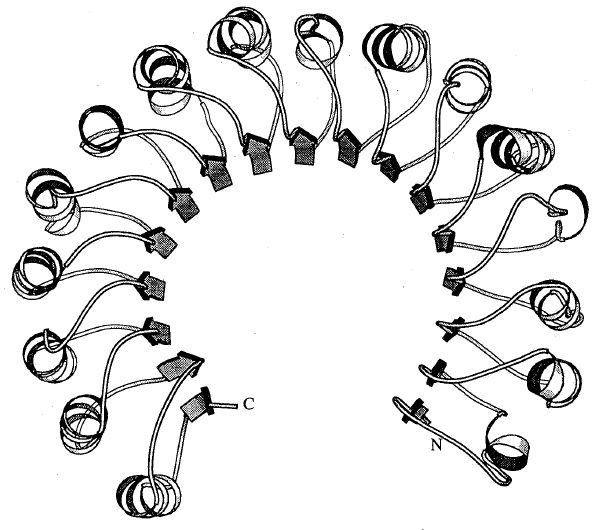 Horseshoe fold made of alternating β strand and α-helices observed in a ribonuclease inhibitor (B. Kobe and J. Deisenhofer, Abst. PS24, pg. 152, ACA Abstracts, Series 2, Vol. 21, May 1993, Albuquerque, NM). Photo courtesy of B. Kobe.
Horseshoe fold made of alternating β strand and α-helices observed in a ribonuclease inhibitor (B. Kobe and J. Deisenhofer, Abst. PS24, pg. 152, ACA Abstracts, Series 2, Vol. 21, May 1993, Albuquerque, NM). Photo courtesy of B. Kobe.
G. Sheldrick's presentation was a model for a plenary lecture. He reviewed recent advances in the implementation of the theory and technique of phase determination and its applications, placed his Patterson award-winning work in that context, challenged the audience with his personal list of new directives for the future of that field, and opened the talk to questions and lively discussion from the floor.
Over 400 posters covering every area of crystallography were on exhibit for the full five days of the meeting. A thumbnail sketch of 45 of the posters in the small molecule single crystal field was presented as three minute talks on Monday morning. This popular format provides an overview of recent achievements in a branch of the discipline, brings new faces to the attention of the community, and stimulates interdisciplinary communication. Other special interest groups are planning to use the format at next year's meeting in Atlanta, GA, June 26-July 1, 1994. The program and local chairmen for Atlanta (C. Carter and L. Williams, respectively) are already pondering the matter of developing a meeting logo that will look as good as the native Indian symbol that graced the program abstract book and T-shirt from the Albuquerque meeting.
Highlights of meeting reports from different divisions of the ACA follow.
 Patterson Award winner George Sheldrick (left) managed to keep his eyes open during the after banquet speech of the ACA Past President Keith Watenpaugh (right). Photo courtesy of W.L. Duax.
Patterson Award winner George Sheldrick (left) managed to keep his eyes open during the after banquet speech of the ACA Past President Keith Watenpaugh (right). Photo courtesy of W.L. Duax.
Theory and Methods
Examples of anomalous scattering in Material Research included demonstrations of the anisotropy of anomalous scattering factors, causing variation in the intensity of a Bragg reflection as the crystal is rotated about the diffraction vector. While these measurements may prove to be a sensitive probe of chemical environment and bonding geometries, their usefulness is currently limited by the absence of any means of predicting these effects for even the simplest crystals. This is a clear challenge to solid state theorists. L. B. Sorenson, E. D. Specht, and Y. Gao discussed application of diffraction anomalous fine structure (DAFS) to select for analysis not just an element but a crystallographic site, one compound in a mixture, one layer in a multilayer film, or atoms segregated to an interface. Conversely, spectroscopic features may be used to locate atoms with selected valence.
In macromolecular crystallography, the exploitation of multiwavelength anomalous diffraction (MAD) methods to solve the phase problem is rapidly developing from a frontier approach to a routine technique. The session highlighted the expanding availability of appropriate experimental facilities; and the re-emergence of phase calculations based on procedures developed for multiple isomorphous replacement (MIR) phasing. A. Aggarwal, V. Ramakrishnan, and J. Martin presented the results of structure determinations of selenomethionine-containing proteins using CHESS station 2, and NSLS stations X12C and X4A for MAD data collection. An overview of the MAD method and applications presented by W. Hendrickson included work in progress where gap-tuned undulator radiation was used at ESRF to exploit the anomalous diffraction of lanthanides. The power of Laue data to reveal subtle molecular features was eloquently demonstrated by R.M. Sweet. The hydrolysis of an acylated trypsin was triggered by a pH jump and the results were followed by three X-ray structure determinations. Comparisons showed that the initial step in the hydrolysis involved the interaction of a single water molecule with the acyl group.
J. Luft et al. presented a new crystallization method involving protein dialysis or vapor diffusion against a very tall reservoir with the precipitant initially a solid lump at the bottom. Badger et al. mapping the distribution of disordered solvent and counterions in large channels in cubic insulin used low resolution anomalous scattering data to locate poorly ordered thallium cations. Three papers by Weeks, Miller, Langs, and Hauptman reported on improvements in the "Shake and Bake" method for determining structures using Hauptman's minimal function. The method was successfully applied to locate the 400 nonhydrogen atoms in crambin using 0.83 Å resolution data. The method is computationally feasible for small proteins.
 Members of the US National Committee for Crystallography at the Albuquerque meeting (left to right, front to back): D. Marsh, J. Griffin, E. Adman, P. Fitzgerald, V. Cody, M. Hackert, A. Eades, J Deisenhofer, H. Berman, D. Cox, S.N. Rao, D. Duchamp, T. Gentile, P. Coppens, J. Kaduk, B. Wuensch, J.Flippen-Anderson, K. Watenpaugh. Chairman R. Bryan too busy to pose.
Members of the US National Committee for Crystallography at the Albuquerque meeting (left to right, front to back): D. Marsh, J. Griffin, E. Adman, P. Fitzgerald, V. Cody, M. Hackert, A. Eades, J Deisenhofer, H. Berman, D. Cox, S.N. Rao, D. Duchamp, T. Gentile, P. Coppens, J. Kaduk, B. Wuensch, J.Flippen-Anderson, K. Watenpaugh. Chairman R. Bryan too busy to pose.
Materials Research
Many friends of the late Bill Parrish gathered to hear a series of papers surveying the state-of-the-art of powder diffractometry - the field he began 50 years ago. Talks by his former postdoctoral students and collaborators described the design of the Parrish-Hart diffractometer (M. Hart), advances in application of new parallel beam diffractometers (M. Eatough), fitting of asymmetric profiles (L. Finger), dynamic analysis techniques (G. Zohrer), and calibration of residual stress (C. Hubbard). D. Smith ended the technical session with a broad survey of the current applications of powder diffraction with an emphasis on the use of whole pattern fitting in qualitative and quantitative analysis and B. Post reminisced about his long association and friendship with Bill. The session was one of the best surveys of powder diffractometry ever presented at an ACA meeting and a very fitting way to honor the memory of William Parrish.
A session on Thin-Film Characterization by Grazing Incidence Diffraction and Reflectometry organized by G. Zorn and E. Ryba revealed how much information can now be obtained by this technique. High resolution diffraction from thin films allows the determination of lattice mismatch, crystalline quality, and chemical stoichiometry. Special reflectivity yields film thickness, roughness, and density and also proves the presence of corrosion layers of several nanometers thickness. Diffuse scattering at grazing incidence produces information on the lateral surface structure (ruggedness of the surface, height to height correlation length), comparable to atomic force microscopy.
Recent advances in techniques for analyzing fiber diffraction data on industrially useful and biologically important materials were the topic of another special session. Fiber diffraction applications ranged from simple model systems to complex nucleic acid polymers.
In a session on Neutron Scattering, A. Kawitz (U. of MO) presented results of residual stress measurements of circumferential welds in a steel casing from a subscale model of an advanced solid rocket booster motor used in NASA's Space Shuttle Program. The "sample," measuring 1 foot tall by 3 feet in diameter and weighing 230 pounds, was studied using neutrons and required fairly precise positioning for the 30 or so points measured along and near the welds. In a session on superconductors S. Billinge described the use of Pair Distribution Functions to determine the local disorder due to oxygen displacements in the Ce-doped Nd2CuO4 material.
Twenty-six poster presentations in the Materials Science field described films and crystals of high temperature superconductors, metal lactates, metal phosphates, deuterated materials, fullerenes, and zeolites. A poster by Vanderah and Lowe-Ma described the effect of the pseudo-cubic rhombohedral distortion of LaAlO3 on the growth and properties of superconducting films. Another poster, by Stoltzberg et al., described the modeling of the dynamics of dendritic crystal growth and the correlation of rapid dendrite growth with chaos theory.
Small Molecule
A special session on Mobility in Molecules began with a review by K. Trueblood of the developments in the use of Bragg and diffuse scattering data in analysis of molecular motion in crystals in the 15 years since the seminal contributions of D.W.J. Cruickshank. A highlight of the session was the presentation by S. Heyes of the uses of solid state NMR in examining various aspects of molecular dynamics in crystals. He described the use of deuterated metallocenes as guest "motional probes" in a wide range of crystalline environments. The potential of the NMR methods for studying phenomena on time scales varying from 10-10 s to 102 s and thus of permitting meaningful clarification of what is meant by "static" and "dynamic" disorder in some crystals was illustrated with many examples, including geared motions, ring flips, and studies of metallocenes in different c1athrates. B. Craven reviewed studies of H-atom motions using neutron diffraction data. After correction for overall rigid-body motion, established with the non-H atoms, the motion of CH2 groups showed remarkable consistency in different compounds and at different temperatures, indicating that the pattern revealed zero-point motion and is transferable.
 ACA Vice President-Elect Ellie Adman, Treasurer Vivian Cody, and Executive Secretary Marcia Vair getting acquainted with the locals at the Albuquerque ACA meeting. Photo courtesy of W.L. Duax.
ACA Vice President-Elect Ellie Adman, Treasurer Vivian Cody, and Executive Secretary Marcia Vair getting acquainted with the locals at the Albuquerque ACA meeting. Photo courtesy of W.L. Duax.
The continued use of NMR and X-ray data to estimate the extent of covalent bonding in Ln+3 complexes was described by A. Pinkerton to open a session on Lanthanides and Actinides in Transition: Catalytic and Biological Perspectives. T. Condari also emphasized the synergism of combining techniques, illustrating the consistency between Molecular Mechanics (MM) calculations using a fairly simple forcefield for a Gd-complex, and the structure of the complex determined from single crystal techniques. These computations augmented the understanding of the electronic and magnetic character in this species. An excellent illustration of the lanthanide contraction was contributed by A. Burrell. Employing an expanded porphyrin with a fixed cavity size he showed that as the Ln was changed from left to right in the series (i.e., decreasing radius) the metal ion dropped into the planar ligand as the match between the cation and cavity improved.
Studies of inorganic, organic, organometallic, and biologically active small molecules presented in poster format included numerous examples of: "the calculation of the electrostatic potentials and their correlation with chemical reactivity or pharmacological activity"; "the relation of molecular conformations derived crystallographically to pharmacological activity"; "the examination of molecular mobility in solids using X-ray analysis or solid-state NMR"; "the resolution of problems presented by twinning and disorder in crystals"; "the analysis of molecular packing in crystals by graph set theory"; "the relationship of the strength and orientation of hydrogen bonds to molecular packing"; "the interplay of packing forces and molecular conformation"; "the formation of salts and co-crystals of biomolecules;" and "the symmetry relationship among multiple molecules in the asymmetric unit".
Specific examples of some of these applications were the use of graph sets to facilitate identification of H-bonded networks necessary for activity in biomolecular systems (M. Kubicki and P.W. Codding), examination of the role of hydrogen bonding in determining packing arrangements (C.P. Brock and J.L. Flippen-Anderson), and modeling anisotropic hydrogen thermal parameters by combining rigid body refinement with standard internal vibrational displacements (S.P. Kamperman et al.). In another poster an equilibrium between a red square plane and a green tetrahedral isomer of a Ni complex was reported by undergraduate E. Rozovi. W. Kwiatkowski and J. Karolak-Wojciechowska calculated the electrostatic potentials of 27 phenylsuccinimides and correlated the numerical difference of electrostatic potential minima located at the two carbonyl oxygen atoms of the imide ring with anti-convulsant activity. Finally, the use of cryogenic data in resolving a problem involving disorder in a Cu ion position was demonstrated (F. Fronczek).
Macromolecular Applications
Macromolecular studies included many of immediate relevance to disease such as the design of inhibitors of glycolytic enzymes in the trypasome, the causative agent in sleeping sickness. An entire poster session was devoted to immune recognition and included structures of Fab antibody fragments against tetanus toxoid, feline infectious peritonitus virus, glycoproteins of human cancer cells, the common cold virus, blood platelet aggregation factors, foot-and-mouth disease virus, and bacterial cell wall oligosaccharides. Information about amyloidosis may come from the comparison of the structures of two Kappa variable chains, only one of which is amyloidogenic. The different ways in which protein phosphorylation can induce physiologically important conformational changes was one of the topics addressed in a session on signal transduction and structural biology. The induction of domain closure around a substrate for a regulated protein kinase (E.G. Goldsmith), the blockage of formation of an inhibitory complex between factor III protein and glycerol kinase (J.H. Hurley), and the creation of a recognition site in the lck kinase SH2 domain by phosphorylation were illustrated. In the same session the actin/profilin structure described by C. Schutt suggests a mechanism by which the SH3-like profilin molecule regulates helix-ribbon transitions in the actin fiber, a quaternary transition that may be central to the mechanism of muscle contraction.
Highlights of the poster session on metallo and electron transfer proteins included a report of a structure of the soluble domain of cytochrome f composed of a β-sandwich and a four-stranded anti parallel β-sheet with the novel feature of a heme iron ligand at the amino terminus (S.E. Martinez). P. Grochulski described two crystal structures of lipases, hydrolytic enzymes that catalyze reactions at oil/water interfaces. These lipase structures differ mainly in the position and conformation of a flap. In the "closed" flap conformation the active site is completely occluded, and the hydrophilic face of this amphipathic flap is exposed to the surface. Conversely, in the "open" flap conformation, the catalytic serine and the hydrophobic surface of the flap are exposed. In adopting the open flap position some residues must move up to 20 Å with respect to the closed state and a proline peptide bond goes from cis to trans.
The poster session on Nucleosides covered double helical DNA with and without bound proteins or drugs, DNA hydration and bending, new structural methods and RNA. The first triclinic space group for a CG-containing decamer was reported by H. Patel and M. Kopka et al. presented the first covalently bound DNA-drug adduct (Anthramycin bound to a decamer). J.M. Harp demonstrated that the use of a palindromic sequence improved the resolution of nucleosome core particles. A poster illustrating the utility of the new Nucleic Acid Database was highly informative. A minitutorial synopsis can be obtained from H. Berman ([email protected]).
This summary of the scientific program of the 1993 ACA Meeting was condensed from the detailed reports of the session chairmen that appear in full in the June 1993 issue of the ACA Newsletter. The contributors were: D. Anderson, L.C. Andrews, J. Badger, M. Becker, J.T. Bolin, R. Bryan, R. Chandrasekaran, W. Cordes, C. Foris, F. Fronczek, D. Ghosh, L. Guddat, J. Hanson, L. Jensen, J. Karle, M.L. Kopka, E. Lingafelter, P. Loll, C. Lowe-Ma, W. Montfort, M.E.P. Murphy, P. Newcomer, G. Newton, W.H. Ojala, R. Reynolds, F.J. Rotella, J. Ruble, T. Rydel, R. Snyder, S. Sommerer, E.D. Specht, S. Sprang, L. Steinrauf, E.A. Sudbeck, A. Swain, S.M. Swanson, K. Trueblood, and G.M. Zorn.


