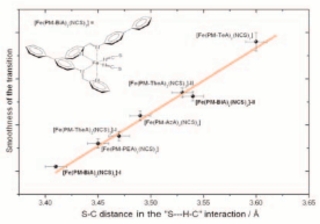
Top left: A supra-molecular self-assembly ruthenium mixed valence (Ri(II)/Ru(III) diphosphine-porphyrin used for the elaboration of modified electrodes. L.R. Dinelli and A.A. Batista1 (U. Federal de São Carlos), E.E. Castellano, and J. Ellena (U. de São Paulo).
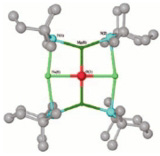
Bottom left: Crystal structure of an enzyme from the tropical parasite Trypanosoma cruzi (that causes an endemic disease in Brazil called Chagas disease) in complex with a natural product inhibitor, isolated from a plant found growing wild in Brazil (courtesy of G. Oliva, LCEPID/FAPESP, de Sao Carlos, U. de Sao Paulo)
Top right: Map highlighting the location of crystallography labs in Latin America
Middle right: The structure of Myelin Basic Protein (MBP), purified from the myelin sheath in both lipid-free (LF-MBP) and lipid-bound (LB-MBP) forms, was investigated in solution by small angle X-ray scattering (SAXS). Under all conditions, the scattering from the two protein forms was different, indicating different molecular shapes. For the LB-MBP, well-defined scattering curves were obtained, suggesting that the protein had a unique, compact (but not globular) structure. The results represent the first direct structural information from X-ray scattering measurements on Myelin Basic Protein in its native lipidic environment in solution. C. L. P. Oliveira, I. L. Torriani (IFGW-Unicamp & LNLS), H. Haas (Munich Biotech), E. Polverini, P. Cavatorta (U. de Parma), A. Fasano, G. Carlone (U. de Bari), P. Riccio (U. of Basilicata). Accepted in Biophysics Journal, September 2003.
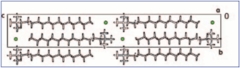
Bottom right: DEI of a spider: (a) on the maximum and (b) on the flank of the rocking curve. The white arrows point out where there are inversion contrasts (black to white) in the image. This cannot be seen in absorption radiographies. Courtesy of Cesar Cusatis, LORXI, U. Federal do Paraná.