


Meeting report
Glasgow Protein Structure Workshop
Sixty participants attended the first Glasgow Protein Structure Workshop at the University of Strathclyde, July 4-6, 1994, with support from Scottish Enterprise, Enraf-Nonius, Mar-Research, and Zeneca Pharmaceuticals.
Keynote speaker, M. Garavito, began the meeting with a lecture on the 3.5 Å structure determination of prostaglandin synthase (Nature 367, 243, 1994 and March photo in the crystallographic calendar), a "monotopic" membrane protein (since it penetrates one side only of the lipid bilayer) which controls the generation of cell-growth hormones and is inhibited by the binding of aspirin and other nonsteroidal anti-inflammatory drugs. The bifunctional enzyme has a cyclo-oxygenase active site close to the membrane and a heme-containing peroxidase site further away. There is also an epidermal growth factor-like domain and a novel membrane-binding domain, consisting of helices which lie almost parallel to the membrane. The enzyme is of interest to pharmaceutical companies wanting to develop anti-inflammatory drugs.
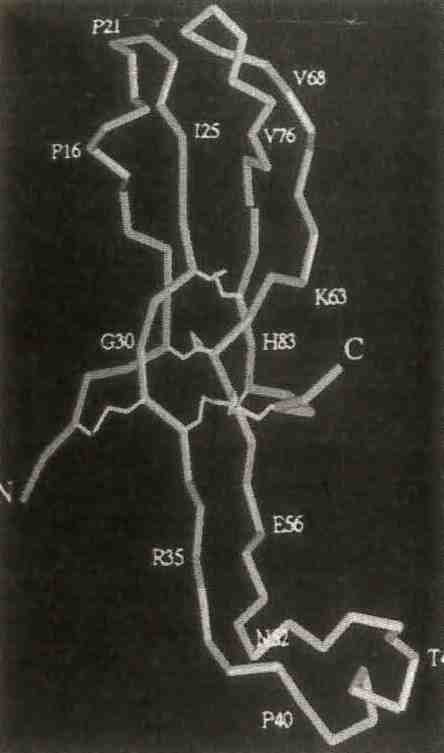 The a subunit of human chorionic gonadotrophin contains 92 amino acids, and 5 disulfide bridges that form cystine knots at the center of the elongated molecules. From "Crystal Structure of Human Chorionic Gonadotrophin," A. L. Lathrom et al., Nature 369, 455, 1994.
The a subunit of human chorionic gonadotrophin contains 92 amino acids, and 5 disulfide bridges that form cystine knots at the center of the elongated molecules. From "Crystal Structure of Human Chorionic Gonadotrophin," A. L. Lathrom et al., Nature 369, 455, 1994.
Garavito presented additional talks on the theory and practice of membrane protein crystallization and his experiences working with prostaglandin synthase (the bottom line seemed to be "know your protein"). He used as much protein over the years characterizing its behavior in the presence of detergents as he has in crystallization trials. In total, he used about 1.5 grams during the course of its structure determination. An overview of membrane protein structures by A. Tucker revealed that all successes with membrane proteins to date involved protein isolated from natural sources. The use of recombinant techniques for the expression of large quantities of membrane protein is not yet routine. Structures of soluble versions or fragments of membrane proteins provided a valuable alternative to structure solution of the intact membrane protein. R. Pauptit and G. McDermott described their experiences with porin and the light-harvesting antenna complexes, respectively.
Other sessions concerned new structure presentations (e.g., human chorionic gonadotrophin, presented by N. Isaacs, a difficult structure eventually solved by the application of the maximum likelihood software MICE; also, the structure of human tissue factor presented by B. Boys), as well as detailed discussions of mechanisms of action of the highly poisonous ricin (S. Weston), glutamate dehydrogenase (L. Britton). There were discussions of penicillin acylase (S. Done); DNA-drug interactions (C. Smith), thermal diffuse scattering (S. Prince), and low temperature studies of gamma-crystallin (V. S. Kumaraswany). A series of presentations on unsolved structures highlighted problems protein crystallographers typically encounter. D. Holden described molecular replacement analysis of a C-reactive protein and N. Glykos presented a Patterson analysis for the Arginine repressor/activator. Finally B. Schierbeek from Enraf-Nonius presented details of the new DIP2000 Image plate detector.
Lindsay Sawyer, U. of EdinburghCondensed from BCA Newsletter No. 51.


