


Meeting report
Membrane proteins: the next frontier
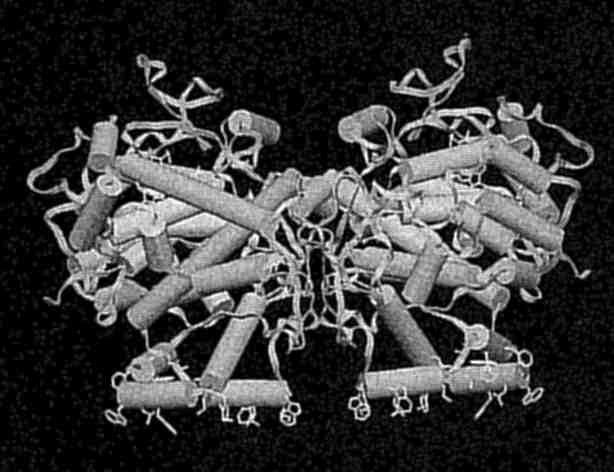 A view of the crystallographically determined structure of prostaglandin synthase, a new membrane protein illustrating the cyclooxygen channel and the active site (Picot et al., Nature, 367, pp. 243-249). (Photo courtesy of M. Garavito)
A view of the crystallographically determined structure of prostaglandin synthase, a new membrane protein illustrating the cyclooxygen channel and the active site (Picot et al., Nature, 367, pp. 243-249). (Photo courtesy of M. Garavito)
On August 16-17, 1993, the National Institute of General Medical Sciences sponsored a workshop on the NIH campus, entitled "Structural Biology of Respiratory Enzymes: Crystallography and NMR of Membrane Proteins". Over 130 scientists participated. The focus of the meeting was on the potential for determining structures of the mitochondrial respiratory enzymes and related enzymes of plant and microbial bioenergetics. However, the points raised should be applicable to many other membrane proteins (e.g., other ion pumps, ion channels, substrate transporters, neurotransmitter uptake systems, and G-protein coupled receptors).
G. Feher presented a talk emphasizing the value of the known bacterial photoreaction center structures to elucidating the function of photosynthetic proteins. J. Deisenhofer presented a review of the membrane protein structures that have been solved by X-ray crystallography. R. Glaeser provided an overview of electron diffraction methods that have been applied to two-dimensional crystals and emphasized the value of pursuing both 2D and 3D crystallization approaches in parallel. W. Cramer chaired a discussion session to review work in progress. It is clear that a limited number of efforts are presently under way.
M. Garavito presented a talk on the lessons learned about crystallization from the few known membrane protein structures and emphasized the need for additional systematic exploration of the many variables involved. He also reported the solution of a new membrane protein structure (i.e. prostaglandin synthase-representing a fourth distinct membrane domain fold). E. Stura chaired a panel discussion on crystallization methods and presented highlights (including crystals) from the Membrane Protein Crystallization Workshop that was held in conjunction with the Fifth International Conference on the Crystallization of Biological Macromolecules in San Diego during the preceding week.
 The IUCr Newsletter Editor attempting to get speakers at the Workshop on Structural Biology of Respiratory Enzymes to relax in front of the camera. From left E. Adman, WLD, J. Deisenhofer, E. Sturo, M. Garavito and M. Anzel. (Photo WLD)
The IUCr Newsletter Editor attempting to get speakers at the Workshop on Structural Biology of Respiratory Enzymes to relax in front of the camera. From left E. Adman, WLD, J. Deisenhofer, E. Sturo, M. Garavito and M. Anzel. (Photo WLD)
E. Adman presented an analysis of the problems likely to be encountered in solving large membrane protein structures. These do not appear to be substantially different from those affecting other structural problems, but may be exacerbated by larger than average unit cell volumes and poorer quality crystals.
S. Ferguson-Miller presented a talk and R. Gennis chaired a panel discussion on protein production issues. Several of the mitochondrial enzymes, or their plant and microbial equivalents, are available in high purity and in large amounts. Some have been cloned and overexpressed, others are from natural sources. Opportunities clearly exist to pursue crystallization of several members of this protein class. A number of transport proteins, including bacterial sugar transporters, mitochondrial membrane ion transporters, and the cystic fibrosis transmembrane regulator, also appear to be promising candidates for crystallization.
Additional speakers and panel members included: M. Amzel, J. Smith, M. Lyon, G. Scarborough, C.-A. Yu, S. Chan, B. Trumpower, T. Ohnishi, J. Fee and P. Pedersen. N. Allewell and R. Kaback served as overall chairpersons.
Major scientific points that were illustrated during the course of the meeting included:
- The value of even the few known membrane protein structures in revolutionizing several fields of research
- The feasibility of obtaining atomic resolution structures for membrane bound proteins
- The ability to produce suitable material for crystallization trials for a variety of energy transducing membrane bound proteins
- Progress toward understanding the intermolecular contacts that are important for producing diffraction quality crystals
- The value of pursuing both 2D crystallization and 3D crystallization approaches in parallel
- The adequacy of current methods to solve the large unit cell structures of membrane proteins, once crystals are in hand
- The value of NMR to provide complete structural solutions for fragments of the proteins of interest and local information for even very large intact proteins in their native membrane environments.
Workshop Recommendations
The workshop concluded with a general discussion session which identified the following needs to facilitate research in membrane protein crystallography:
(1) Tools
(a) Further development of methods for overexpression of native and modified membrane proteins; (b) high purity detergents, novel detergents, and non detergent surfactants for purification, stabilization and crystallization of membrane proteins; (c) novel reagents and approaches for heavy atom derivatization; (d) novel techniques and hardware for crystallization and for the manipulation of fragile crystals, including apparatus to facilitate temperature control.
(2) Facilities
(a) Increased access to synchrotron X-ray beamlines; (b) improved biochemical support lab facilities at synchrotron sites; (c) funds for travel to data acquisition centers.
(3) Methodological developments
(a) Basic research on the physics of membrane protein crystallization and on crystallization in general; (b) further development of methods for electron diffraction and especially methods for production of 2D crystals.
(4) Training
(a) Renewed interest and training in the protein chemistry of membrane proteins-purification, characterization and stabilization; (b) increased training in the methods of protein crystallization.
(5) Research funding
(a) Stable funding for projects requiring reasonably long-term efforts, including both method development projects and crystallization efforts; (b) support for collaborations between crystallographic labs and protein chemists/molecular biologists with interests and experience in handling membrane proteins.
(6) Communications
Additional vehicles for dissemination of work in progress, including informative failures.
Thus far NIGMS has taken the following steps to stimulate research on membrane protein structures: (a) Issue a Special Emphasis Announcement for the Small Business Innovation Research (SBIR) Program which included the areas of expression systems, detergents, crystallization apparatus, and heavy atom derivatization reagents [See: Omnibus Solicitation of the Public Health Service for Small Business Innovation Research Grant Cooperative Agreement Applications (94-3 and Special Supplement 93-2S)]; (b) include basic research on the principles of protein crystallization, particularly for membrane and glycosylated proteins, within an AIDS Program Announcement for regular research grants [See: NIH Guide. vol. 22 (No. 43). Nov. 26, 1993, PA 94-014]; (c) indicate willingness to support a hands-on course which would help more investigators to begin working on membrane protein crystallization; (d) indicate willingness to consider membrane protein crystallization efforts under a general plan to support high risk, high payoff research.
A complete copy of the workshop report is available by contacting Dr Peter C. Preusch at NIGMS (301-594-7806). The National Institute of Diabetes, Digestive, and Kidney Diseases has also expressed interest in membrane protein structural biology. Contact Dr Eliezar Dawidowicz for further information (301-594-7582).
P. Preusch(NIH, USA)


